- High Purity Sucrose Ester Of Fatty Acids
- Sucrose Ester Of Fatty Acids S-CM
- Sucrose Ester Of Fatty Acids
- Compound Food Additive
- 2,3,4,6-Tetra-O-Benzyl-D-Glucopyranose
- 2,3,4,6-Tetra-O-Benzyl-D-Galactose
- Mono-Acetone-D-Glucose
- Diacetone-D-Glucose
- Methyl α-D-Mannopyranoside
- 1,2,3,4-Tetra-O-acetyl-β-D-glucuronic acid methyl ester
Carbohydrate Derivative
2,3:5,6-Di-O-isopropylidene-a-D-mannofuranose
Synonym: 2,3:5,6-Di-O-isopropylidene-a-D-mannofuranose
Molecular Formula: C12H20O6
Molecular weight: 260.29
CAS No.: 14131-84-1
Molecular structure:
.png)
Quality Standard:
|
Item |
Specification |
|
Appearance |
White to off-white crystalline powder |
|
Assay , % |
≥98.0 |
|
Specific rotation , (C=1,acetone) |
+22.0~ +26.0° |
|
Loss on drying, % |
≤1.0 |
|
Residue on ignition, % |
≤0.10 |
It is used as medical intermediate, the following products can be synthesized:
1.Used for the synthesis of N-alkylated polyhydroxylated pyrrolidine compounds, which has a high inhibitory effect on different kinds of glycosidase. It can be used as active components in the preparation of various drugs related to glycosidase diseases. Such as diabetes, Gaucher's disease, tumors and viral infections.
2.Synthesis of sulfur-containing thick and mixed tetracyclic azasugar derivative. It has stronger HIV (Human Immunodeficiency Virus) inhibitory activity, and can be applied in anti-HIV medicinal preparation.
3.Synthesis of the compounds petrosiols A-E,they have the potential to treat Alzheimer's disease and Parkinson's disease.
4. Synthesis of β-C-Glycosides, then synthesis of(−)-Orthodiffenes A and C. It has good anti-tumor activity. These compounds showed significant inhibitory activity against hL-60 and Jurkat cancer cells.
It is also used in synthesize for below products:
1.Synthesis of N-homobicyclic dideoxynucleoside analogues.
2.Synthesis of C-aryl furanoside as a conformationally constrained CHIR-090 analogue.
3.Synthesis of 3‑deoxy‑D‑manno-2-octulosonic acid (Kdo) and its derivatives.
4.Synthesis of P1,P2 –diglycosyl,P1,P1,P2 –triglycosyl, and P1,P1,P2,P2–tetraribosyl methylenediphosphonates.
5.Synthesis of Galactosyl-Queuosine .
6.Synthesis of 4 ' -thioglycosyl donor,then synthesis of truncated N 6 -(3- halobenzyl)amino derivatives.
7. Synthesis of truncated 4 '-thioadenosine derivatives at the human A adenosine receptors.
8. Synthesis of phosphorylated derivative, then synthesis of D -glycero- b - D -manno-heptose 1,7-biphosphate.
9. Synthesis of aminocytitols by Petasis-Borono-Mannich reaction: synthesis of (+)-Conduramine E and (−)-Conduramine E. This synthetic method provides the basis for stereoselective synthesis of several conduramines from carbohydrates.
10. Selective synthesis of (+)-norrisolide. These studies establish the absolute stereochemistry of this natural product and pave the way for a more precise study of its structure–activity relationship.
11. Synthesis of butenolides through an orner−Wadsworth−Emmons Cascading Dimerization Reaction.
12. Synthesis of (-)-isoaltholactone.
13. Synthesis of ethyl (E)-6-hydroxy-4,5; 7,8-di-O-isopropylidene-2-octenoate.
14. Synthesis of non-labeled and 13 C-labeled L–fucoses.
 中文版
中文版 English
English 日本語
日本語 Français
Français Pусский
Pусский España
España

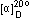 (
( Mobile version
Mobile version Micro station
Micro station